Basic Info.
Product Description

Closed Suction System Single Lumen 24 Hours/ Disposable Medical Closed Suction Catheter For Adult With ISO&CE Certificates
-Closed Suction system is an advanced Closed Suction System.
- It is designed with a protective sleeve to isolate the germs inside and help caregivers avoid cross-infection.
-Simultaneously ventilation design keeps the patients comfortable during suction without stopping the air ventilation.
-Color-coded rings provide fast recognition.

Feature
- Continuous Breathing
Simultaneously ventilation
- Soft Blue Suction Tip
This design reduces damage to the patient while suctioning.
After returning the catheter, the tip of the tube is easier for irrigational cleaning.
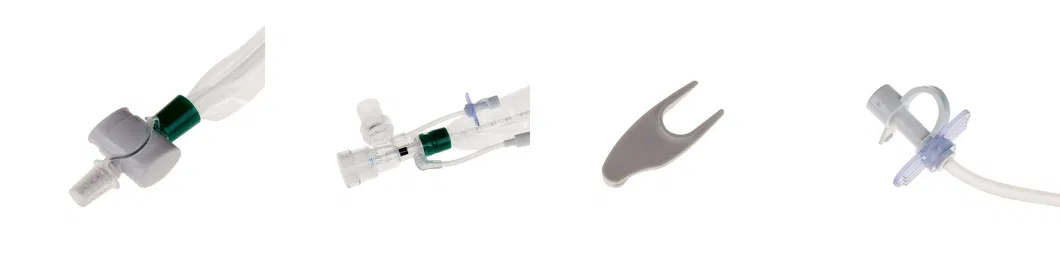
- Double Swivel Connector
15mm connector of Double Swivel
- Disconnecting Wedge
Designed to equip the wedge with both disconnecting and clipping functions
- For Endotracheal Tube and Tracheostomy Tube
Designed for endotracheal and tracheostomy tube using
Tubes with different length are available
- Prevent Cross Infection
Designed with a protective sleeve to isolate the bacteria inside and help caregivers avoid cross-inflection.
Packaging & Delivery
-Packaging Details
-Packing: 1pc/sterilized pouch, 10pcs / inner box, Outer packing: 100pcs / shipping carton
-Delivery Time: Within 20 days. It depends on the quantity of order
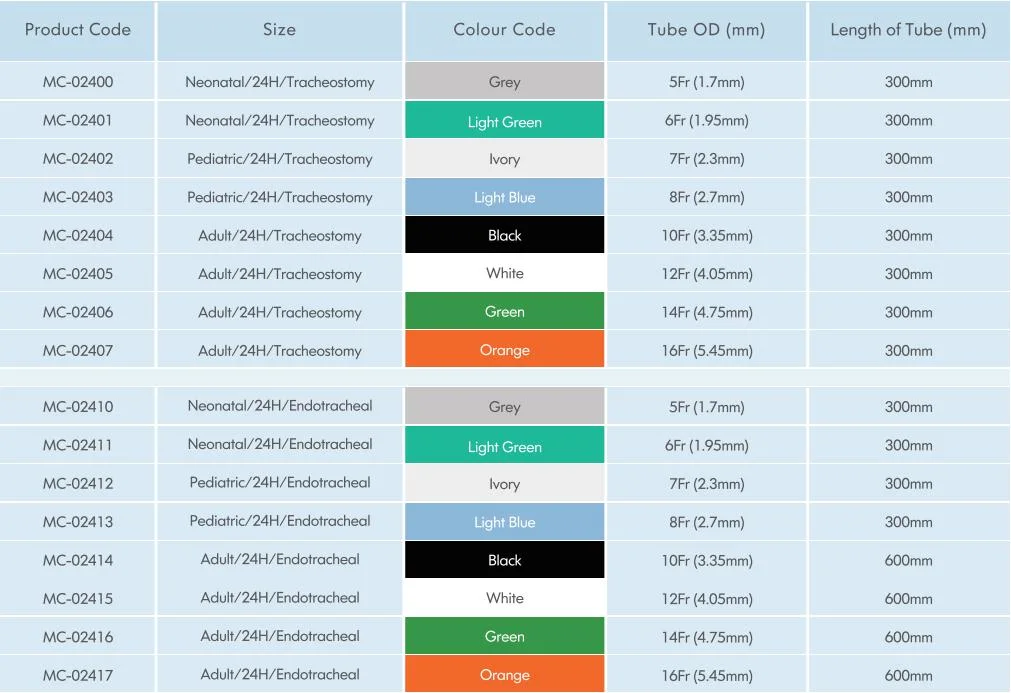
Product Description
Closed Suction Catheter Standard Form
We can produce the closed suction catheter according to customers' requirements!!!
-Instructions for Use
*Set up Procedure
1. Inspect the product before use. Do not use it if the package is not intact.
2. Open the sealed package and take out the product.
3. Connect the Endotracheal Tube/ Tracheostomy Tube to the Revolvable adapter.
4. Connect ventilator tube to the Revolvable Ventilator Connector.
5. Attach the date label to the color ring.
6. Before suctioning commences ensure that the cap of the MDI Port/ irrigation Port is closed.
7.Before suctioning: Please ensure that the on-off valve is in the open position. Simply slide the on-off valve to a position which allows the catheter to enter the Endotracheal tube/ Tracheostomy tube.
*Suctioning Procedure
Caution--- Always use the recommended vacuum levels./ Consider length of suctioning time
1.Grip the three-way adaptor in one hand and with the other hand feed the suction
a catheter into the Endotracheal tube/ Tracheostomy tube to the required depth.
Depth markers can be visible through the protective cover for your guidance.
2.Once the suction catheter is in the desired position/ depth, depress the vacuum control valve to apply suction.
3. Remove the suction catheter until the protective sleeve is straight.
4.Repeat step1-3 as necessary.
Company profile
MCREAT has 16 years' experience in manufacturing medical intubation products. In terms of product technology and production, it is very mature and perfect. Since the World Anesthesia Conference in Amsterdam in 2019, I have talked with a German expert about the future direction of intubation-visualization of all intubation products is the future. Since 2020, the company has developed a series of products such as visual double-lumen bronchial intubation, visual laryngeal mask airway and visual obturator. In the future, the company's goal is to visualize all the inserted catheters (inserted into the body) in urology, gynecology, gastroenterology, etc. At present, the fiberscope and endoscope, which are expensive in hospitals, are made into disposable products. Avoid complete cross-infection, easy to use, protect doctors and patients.








1.Q: Where is your factory located?
A: Our factory is located in Guangzhou city, Guangdong Province, China. There are about 80 workers in our factory. We still try to enlarge our capacity. It is about 30 minutes from Guangzhou South Railway Station. Warmly welcome to visit our factory.
2.Q: Do you provide samples? Is it free or extra?
A: Yes, we can provide samples for free. Welcome to ask us for a sample if you need it.
3.Q: What is your warranty on your products?
A: 4-5 years quality warranty.
4.Q: How long is your delivery time?
A: Generally it is about 30 days, or it is according to quantity.
5. Q: How could we pay for you?
A: Usually By T/T, or other payments can be negotiable.
6.Q: Do you have any certificates of the items you have?
A: Yes, we have got CE0123, ISO13485, CFDA, and FDA approved for main products.
7.Q: Does this product support customization?
A: Yes, we have an R&D team for new product innovation. We will do our best to achieve your goal.
8.Q: How is your after-sale?
A: Any questions, please feel free to contact us. We have a strict Q&A system. I believe that we have responsibility for the after-sale.